The PITHIA trial – a novel trial to improve kidney utilisation
Soon to start recruiting in the UK, the Pre-Implantation Trial of Histopathology In renal Transplant Allografts (PITHIA) trial blends national collaboration, a unique digital pathology service, registry-based follow-up and a novel stepped-wedge design to assess the impact of a national donor histopathology service on kidney utilisation.
The trial is being run by chief investigator Gavin Pettigrew from Cambridge University and the NHSBT Clinical Trials Unit, and will recruit in 22 UK renal transplant centres. The stepped-wedge design will randomise transplant centres in clusters to gain access to a pre-implantation histopathology service for kidneys from donors over 60 years of age. At the start of the trial, no centres will have access to histopathology, and by the end all centres will have access. Analysis will enable us to determine whether access to histopathology for these elderly kidneys improves utilisation and/or outcomes.
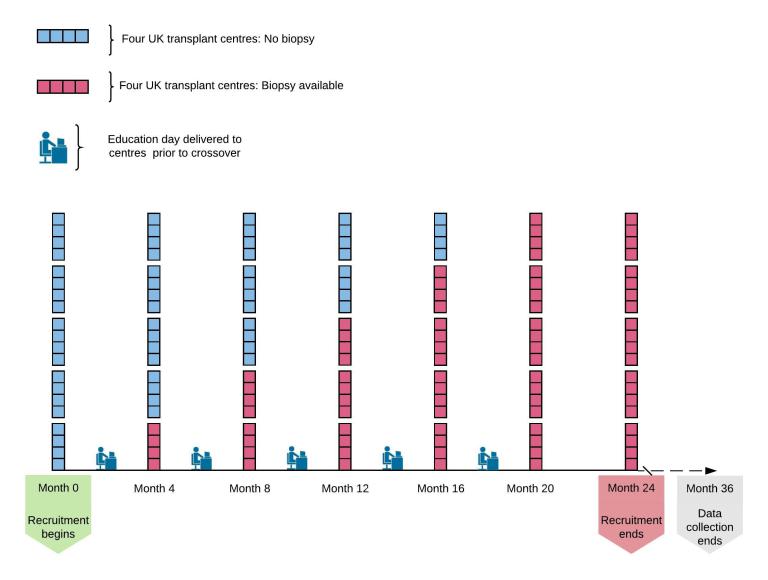
Follow-up for the trial will be entirely registry-based, using routinely collected NHSBT registry data. This means that there will be no additional hospital visits or tests for transplant recipients.
The trial will also use the first national digital histopathology service. Slides from donor kidneys will be scanned by a biomedical technician, and uploaded to a secure online pathology server. The team of trial histopathologists will then be able to read and report the slides from the comfort of their own home, providing national cover from one place.
We are very excited to be involved in this novel trial, and hope that we are able to confirm the excellent pilot results from Cambridge with increased utilisation of kidneys from this cohort of donors.
For more information, please visit the PITHIA website.
